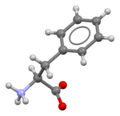
Phenylalanin
 Skeletal formula of L-phenylalanine
| |||
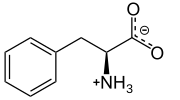 L-Phenylalanine at physiological pH
| |||
| |||
| Hō-miâ | |||
|---|---|---|---|
| Pronunciation | US: /ˌfɛnəlˈæləniːn/; UK: /ˌfiːnaɪl-/ | ||
| IUPAC hō-miâ
Phenylalanine
| |||
| Hē-thóng-tek IUPAC hō-miâ
(S)-2-Amino-3-phenylpropanoic acid | |||
| Sek-pia̍t-hō | |||
3D model (JSmol)
|
|||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.517 | ||
| |||
| KEGG |
| ||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Sèng-chit | |||
| C9H11NO2 | |||
| Mole chit-liōng | 165.19 g·mol−1 | ||
| Acidity (pKa) | 1.83 (carboxyl), 9.13 (amino)[2] | ||
| Gûi-hiám | |||
| NFPA 704 | |||
Tû-liáu te̍k-pia̍t chí chhut, chu-liāu sī kun-kù bu̍t-chit ê piau-chún chōng-thài (tī 25 °C [77 °F], 100 kPa). | |||
| Infobox chham-chiàu | |||

Phenylalanine (siok-siá: Phe / F), sī chi̍t chióng an-ki-sng, R group bô ke̍k-sèng, toà tiong-sèng tiān. Jîn-thé bô-hoat-tō ka-tī ha̍p-sêng chit khoán an-ki-sng.
Tsù-kái[siu-kái | kái goân-sí-bé]
- ↑ 1.0 1.1 Ihlefeldt, Franziska Stefanie; Pettersen, Fredrik Bjarte; von Bonin, Aidan; Zawadzka, Malgorzata; Görbitz, Prof. Carl Henrik (2014). "The Polymorphs of L‐Phenylalanine". Angew. Chem. Int. Ed. 53 (49): 13600–13604. doi:10.1002/anie.201406886. PMID 25336255.
- ↑ Dawson RM, et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
| |||||||
| Pún bûn-chiuⁿ sī chi̍t phiⁿ phí-á-kiáⁿ. Lí thang tàu khok-chhiong lâi pang-chō͘ Wikipedia. |