Durvalumab
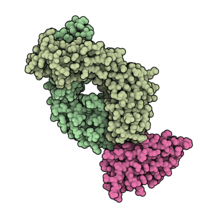 Durvalumab (tshiò-li̍k sik) kah PD-L1 ho̍k-ha̍p ê khòng-guân kiat-ha̍p phìnn-tsat (hún-âng sik). PDB: 5X8M. | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | CD274 |
| Clinical data | |
| Trade names | Imfinzi |
| Synonyms | MEDI4736, MEDI-4736 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617030 |
| data |
|
| Pregnancy category | |
| Routes of administration | Tsīng-me̍h tī-liâu |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6502H10018N1742O2024S42 |
| Molar mass | 146,322.36 g·mol−1 |

Durvalumab (piau-pà PD-L1)[6] í Imfinzi phín-pâi siau-siū, sī FDA phue-tsún ê gâm-tsìng bián-i̍k liâu-huat, iû Medimmune/AstraZeneca sóo khai-huat.[7]Durvalumab sī tsi̍t-t-tsióng jîn-luī bián-i̍k kiû-nn̄g-pe̍h G1 kappa (IgG1κ) tuann clone khòng-thé, ē-tàng tsóo-tn̄g tîng-sū-sìng sè-pau sí-bông phuè-thé 1 (PD-L1) hām PD-1 (CD279) ê hōo-siong tsok-iōng. Durvalumab sī tsi̍t-tsióng bián-i̍k kiám-tsa-tiám ik-tsè-tsè io̍h-bu̍t.[8]
Tsù-kái[siu-kái | kái goân-sí-bé]
- ↑ "Durvalumab (Imfinzi) Use During Pregnancy". Drugs.com. 30 August 2019. goân-loē-iông tī 29 August 2021 hőng khó͘-pih. 7 February 2020 khòaⁿ--ê.
- ↑ "Imfinzi (AstraZeneca Pty Ltd)". Therapeutic Goods Administration (TGA). 5 December 2022. goân-loē-iông tī 18 March 2023 hőng khó͘-pih. 9 April 2023 khòaⁿ--ê.
- ↑ "Regulatory Decision Summary - Imfinzi". Health Canada. 23 October 2014. goân-loē-iông tī 7 June 2022 hőng khó͘-pih. 6 September 2022 khòaⁿ--ê.
- ↑ "Imfinzi EPAR". European Medicines Agency (EMA). 17 September 2018. goân-loē-iông tī 28 August 2021 hőng khó͘-pih. 30 September 2020 khòaⁿ--ê.
- ↑ "Imfinzi- durvalumab injection, solution". DailyMed. 5 June 2020. goân-loē-iông tī 28 August 2021 hőng khó͘-pih. 30 September 2020 khòaⁿ--ê.
- ↑ World Health Organization (2014). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 112" (PDF). WHO Drug Information. 28 (4). goân-loē-iông (PDF) tī 28 August 2021 hőng khó͘-pih. 6 September 2022 khòaⁿ--ê.
- ↑ "Durvalumab (Imfinzi)". U.S. Food and Drug Administration (FDA). goân-loē-iông tī 8 May 2017 hőng khó͘-pih. 2017-05-06 khòaⁿ--ê.
- ↑ Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
Tsham-ua̍t[siu-kái | kái goân-sí-bé]
- CTLA-4
- LAG-3
- Atezolizumab (Tecentriq; target PD-L1)
- Avelumab (Bavencio; target PD-L1)
- Pembrolizumab (Keytruda; target PD-1)
- Nivolumab (Opdivo; target PD-1)
- Cemiplimab (Libtayo; target PD-1)
- Ipilimumab (Yervoy; CTLA-4 inhibitor)
- Tremelimumab (Imjuno; CTLA-4 inhibitor)
- Relatlimab (LAG-3 inhibitor)
Guā-pōo liân-kiat[siu-kái | kái goân-sí-bé]
- "Durvalumab". NCI Drug Dictionary. National Cancer Institute.
- "Durvalumab". National Cancer Institute. 5 May 2017.
- Immune Checkpoint Inhibitors
- Immune Checkpoint Inhibitors and Their Side Effects
- 【2018諾貝爾生醫獎】癌症免疫療法新突破
| ||||||||||||||||||||||
Lūi-pia̍t:
- Drugs with non-standard legal status
- Chemicals that do not have a ChemSpider ID assigned
- Articles without EBI source
- Articles without InChI source
- Articles containing unverified chemical infoboxes
- Drugs that are a monoclonal antibody
- Breakthrough therapy
- Antineoplastic drugs
- Monoclonal antibodies
- AstraZeneca brands
- Orphan drugs
